Abstract
Gadolinium based contrast agents (GBCA) are used in magnetic resonance imaging (MRI) in order to improve the detection and the characterization of pathophysiologic processes. Many studies have reported an association between increased signal intensities (SI) on unenhanced T1-weighted MRI images in different brain regions and the history of repeated intravenous administrations of GBCAs in patients with normal renal function. We conducted a prospective study aimed to evaluate signal changes in the dentate nucleus (DN), globus pallidus (GP), pons, and thalamus (normalized to the deep cerebellum white matter) in T1-MRI images after serial injections of Gadobutrol for the study of the heart in patients with thalassemia.
Among the thalassemic patients who underwent a brain MRI within a pilot study for iron overload assessment, we selected two groups of patients: 15 patients transfused and chelated with≥4 Gadobutrol administrations at a high dose (0.2 mmol/Kg per scan) and 8 thalassemia patients who never received Gadobutrol. Moreover, we included 13 healthy subjects who never received Gadobutrol as control group. Iron overload was assessed by the T2* technique.
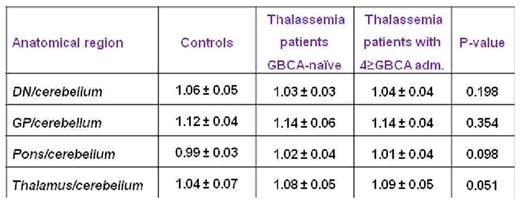
Signal intensity (SI) ratios in all regions were comparable among the three groups (see Table) and were not correlated with the number of Gadobutol administrations. No correlation was detected between SI ratios and T2* values.
It is important to underline that thalassemia patients represent an high-risk population for the GBCA accumulation, due to the fact that, in presence of siderosis, ferric ions can compete with Gadolinium ions for the ligand, destabilizing the gadolinium complex. Nevertheless, our study describes the lack of increased SI after repeated administration of Gadobutrol, a nonionic macrocyclic agent. All our patients were chelated when they received the GBCA. Different studies showed the ability of all three iron chelators to chelate other metals. So, a potential role of chelation therapy may be supposed.
Pepe: Chiesi Farmaceutici and ApoPharma Inc.: Other: Alessia Pepe is the PI of the MIOT project, that receives no profit support from Chiesi Farmaceutici S.p.A. and ApoPharma Inc.
Author notes
Asterisk with author names denotes non-ASH members.